thescipub.com2
American Journal of Biochemistry and Biotechnology 6 (3): 231-238, 2010 ISSN 1553-3468 © 2010 Science Publications Uterus-Relaxing Study of a Sudanese Herb (El-Hazha) 1Aimun Abdelgaffar Elhassan Ahmed, 1Robert Gaspar, 1Arpad Marki, 3Andrea Vasas, 2Mahmoud Mudawi Eltahir Mudawi, 3Judit Hohmann and 1George Falkay 1Department of Pharmacodynamics and Biopharmacy, Faculty of Pharmacy, Universi
 INDUCTION MECHANISMS FOR L-LTP AT THALAMIC INPUT SYNAPSES
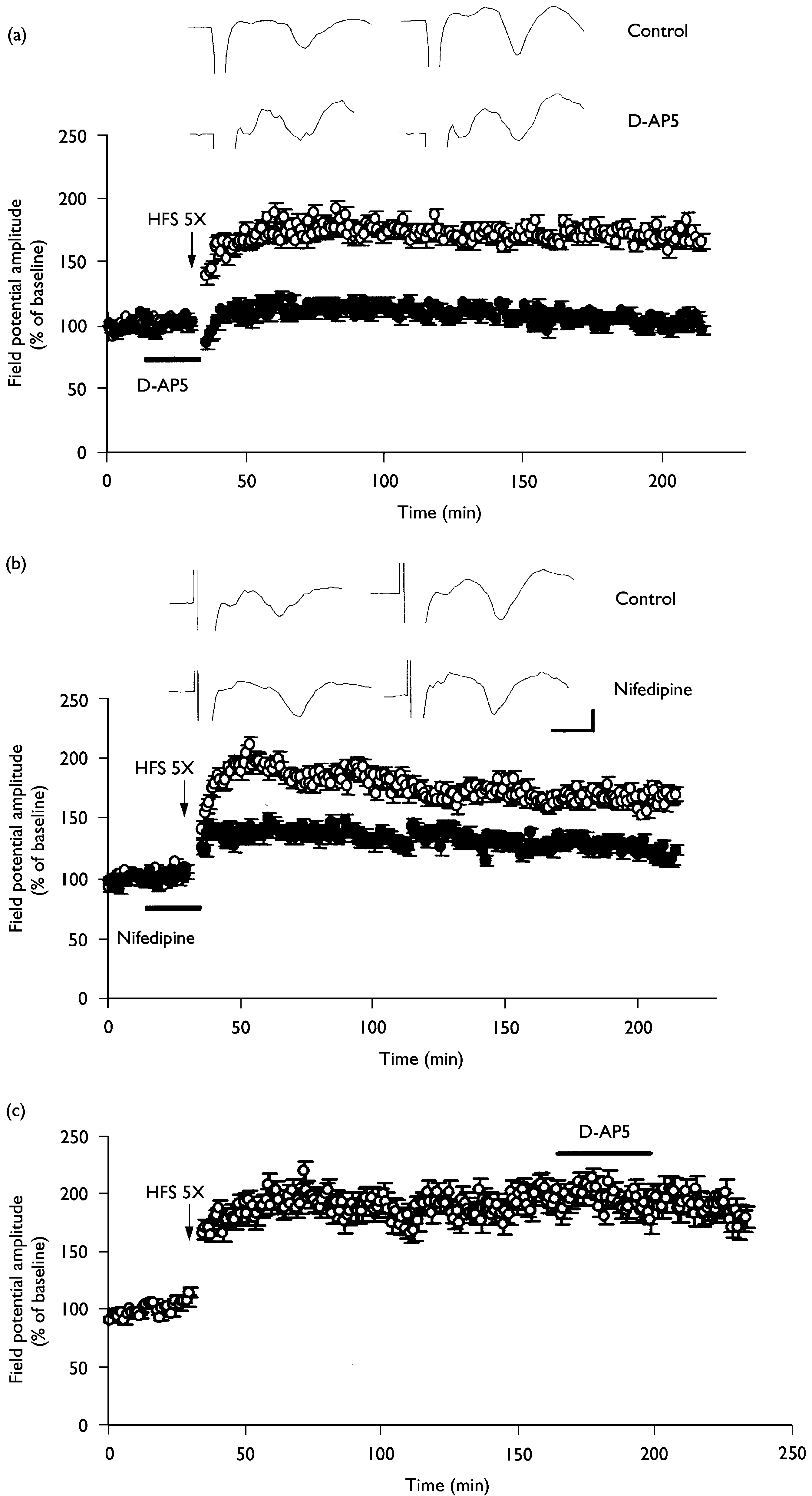
Involvement of NMDA receptors and L-type voltage-gated calcium channels in the L-LTP induction. (a) L-LTP at thalamic input synapses onto the
lateral amygdala was completely blocked by the NMDA receptor antagonist D-AP5 (50 mM, n ¼ 6; closed circles). (b) L-LTP at thalamic input synapsesonto the lateral amygdala was partially inhibited by the L-type voltage-gated calcium channel inhibitor nifedipine (30 mM, n ¼ 6; closed circles). Please notethat L-LTP was maintained even in the presence of nifedipine. (c) The potentiated synaptic responses during L-LTP were not altered by D-AP5 (50 mM,n ¼ 6).The averaged data traces taken before (left) and 3 h after (right) tetanus were shown at the top of the ¢gure. Calibration ¼ 3 ms, 0.2 mV.
INDUCTION MECHANISMS FOR L-LTP AT THALAMIC INPUT SYNAPSES
Involvement of NMDA receptors and L-type voltage-gated calcium channels in the L-LTP induction. (a) L-LTP at thalamic input synapses onto the
lateral amygdala was completely blocked by the NMDA receptor antagonist D-AP5 (50 mM, n ¼ 6; closed circles). (b) L-LTP at thalamic input synapsesonto the lateral amygdala was partially inhibited by the L-type voltage-gated calcium channel inhibitor nifedipine (30 mM, n ¼ 6; closed circles). Please notethat L-LTP was maintained even in the presence of nifedipine. (c) The potentiated synaptic responses during L-LTP were not altered by D-AP5 (50 mM,n ¼ 6).The averaged data traces taken before (left) and 3 h after (right) tetanus were shown at the top of the ¢gure. Calibration ¼ 3 ms, 0.2 mV.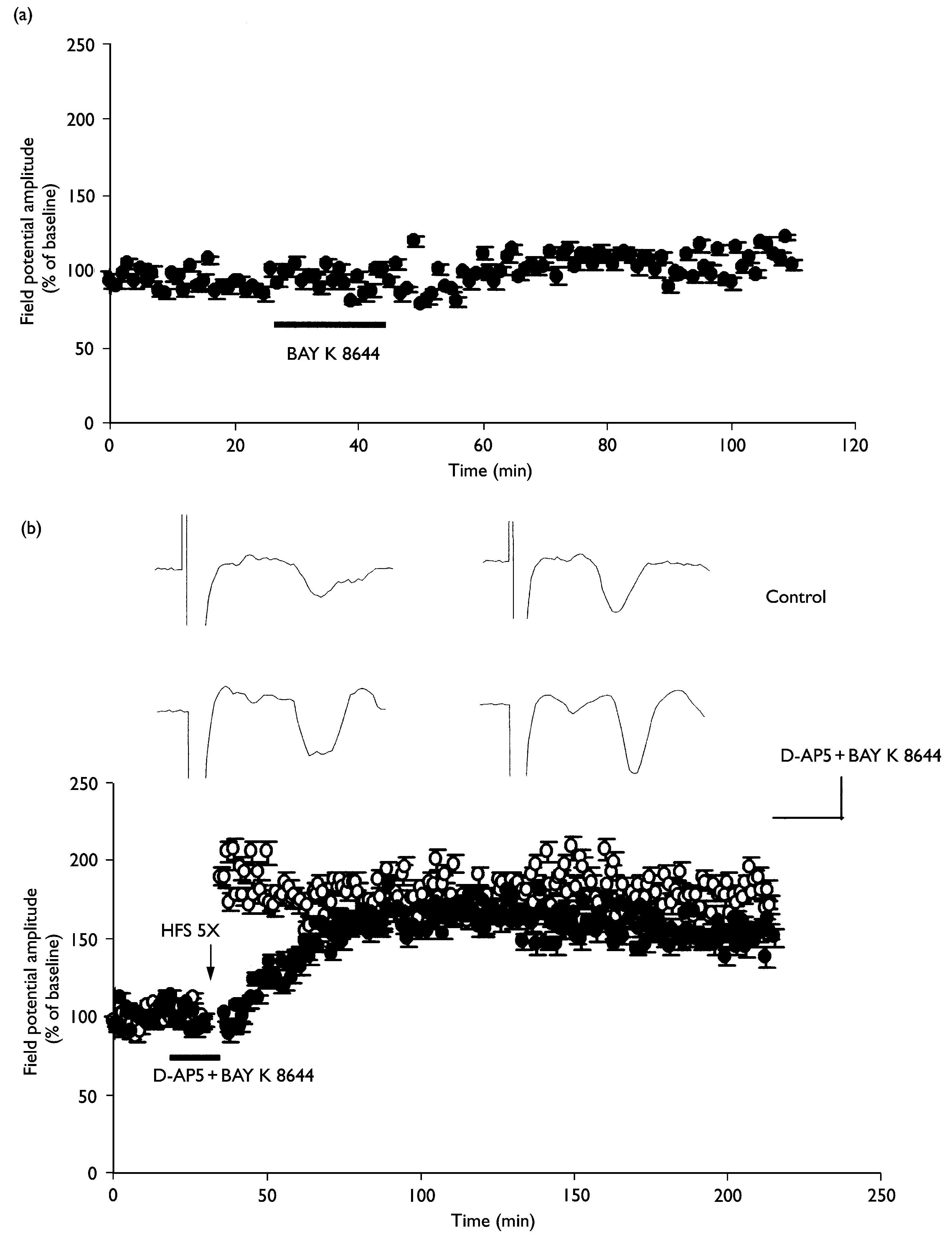 INDUCTION MECHANISMS FOR L-LTP AT THALAMIC INPUT SYNAPSES
Restoration of L-LTP by exposure to BAY K 8644 in the presence of D-AP5. (a) BAY K 8644 (1 mM) alone did not alter baseline synaptic transmis-
sion (n ¼ 3). (b) L-LTP was induced by repeated tetanus before and during exposure to 1 mM BAY K 8644 and 50 mM D-AP5 (n ¼ 4; closed circles). Pleasenote that the potentiation after repeated tetanus develops slowly. The averaged data traces taken before (left) and 3 h after (right) tetanus are shown atthe top of the ¢gure. Calibration ¼ 4 ms, 0.2 mV.
INDUCTION MECHANISMS FOR L-LTP AT THALAMIC INPUT SYNAPSES
Restoration of L-LTP by exposure to BAY K 8644 in the presence of D-AP5. (a) BAY K 8644 (1 mM) alone did not alter baseline synaptic transmis-
sion (n ¼ 3). (b) L-LTP was induced by repeated tetanus before and during exposure to 1 mM BAY K 8644 and 50 mM D-AP5 (n ¼ 4; closed circles). Pleasenote that the potentiation after repeated tetanus develops slowly. The averaged data traces taken before (left) and 3 h after (right) tetanus are shown atthe top of the ¢gure. Calibration ¼ 4 ms, 0.2 mV.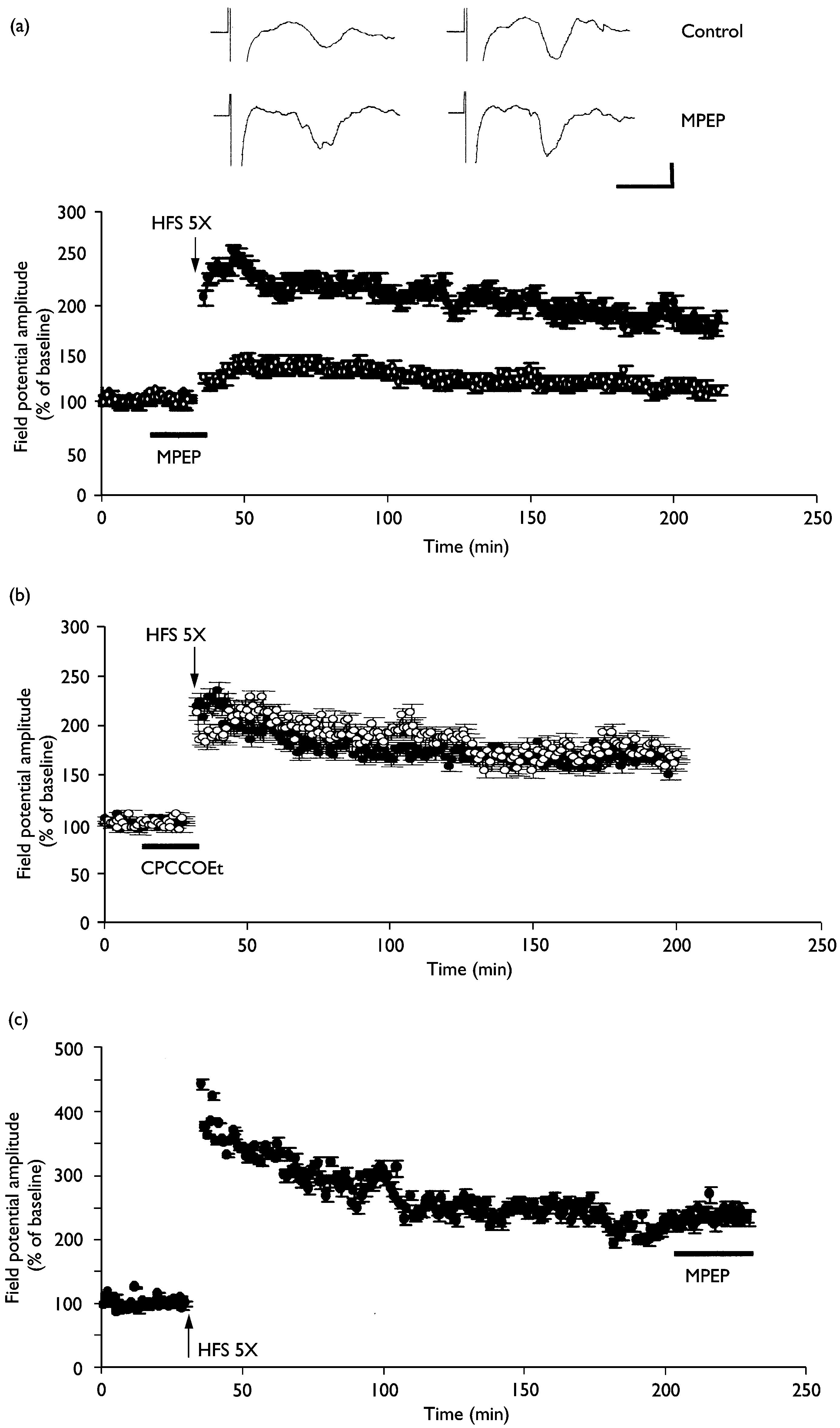 Involvement of mGluR5, but not mGluR1, in L-LTP induction. (a) L-LTP at thalamic input synapses onto the lateral amygdala was completely
inhibited by the mGluR5 inhibitor MPEP (10 mM, n ¼ 5; open circles). (b) The mGluR1 antagonist CPCCOEt (80 mM, n ¼ 5; open circles) failed to block L-LTP induction at thalamic input synapses onto the lateral amygdala. (c) The potentiated synaptic responses during L-LTP were not altered by MPEP (10 mM,n ¼ 3).The averaged data traces taken before (left) and 3 h after (right) tetanus are shown at the top of the ¢gure. Calibration ¼ 5 ms, 0.2 mV.
Involvement of mGluR5, but not mGluR1, in L-LTP induction. (a) L-LTP at thalamic input synapses onto the lateral amygdala was completely
inhibited by the mGluR5 inhibitor MPEP (10 mM, n ¼ 5; open circles). (b) The mGluR1 antagonist CPCCOEt (80 mM, n ¼ 5; open circles) failed to block L-LTP induction at thalamic input synapses onto the lateral amygdala. (c) The potentiated synaptic responses during L-LTP were not altered by MPEP (10 mM,n ¼ 3).The averaged data traces taken before (left) and 3 h after (right) tetanus are shown at the top of the ¢gure. Calibration ¼ 5 ms, 0.2 mV.