03-5202 dep sprinkles.pdf
This information is intended for U.S. residents only. DEPAKOTE® Sprinkle Capsules DIVALPROEX SODIUM COATED PARTICLES IN CAPSULES BOX WARNING: HEPATOTOXICITY: HEPATIC FAILURE RESULTING IN FATALITIES HAS OCCURRED IN PATIENTS RECEIVING VALPROIC ACID AND ITS DERIVATIVES. EXPERIENCE HAS INDICATED THAT CHILDREN UNDER THE AGE OF TWO YEARS ARE AT A CONSIDERABLY INCREASED RISK OF DEVELOPING FATAL
 17a-Ethinylestradiol: AnEndocrine Disrupter of GreatConcern. Analytical Methodsand Removal Processes Appliedto Water Purification. A ReviewLudiwine Clouzot,a Benoıˆt Marrot,a Pierre Doumenq,b Nicolas Rochea
a LM2P2, UMR CNRS 6181, Laboratoire de Me´canique, Mode´lisation et Proce´de´s Propres, Universite´ PaulCe´zanne Aix-Marseille 3, Europoˆle de l’Arbois, Baˆt Lae¨nnec hall C BP 80, 13545 Aix-en-provenceCedex 4; benoit.marrot@univ-cezanne.fr (for correspondence)
b SCC-F-QE, UMR 6171, Laboratoire de Chimie Analytique de l’Environnement, Universite´ Paul Ce´zanneAix-Marseille 3, Europoˆle de l’Arbois, Baˆt Villemin, BP 80, 13545 Aix-en-provence Cedex 4
Published online 16 June 2008 in Wiley InterScience (www.interscience.wiley.com). DOI 10.1002/ep.10291
The xenobiotic 17a-ethinylestradiol, an oral con-
ing microorganisms that are responsible for EE2 bio-
traceptive component, is an endocrine disrupter
degradation. Among bioprocesses, i.e., AS, membrane
(EDC) of great concern, with fish feminization
bioreactors (MBRs), biofilm reactors, and sequencing
induced for concentrations as low as ng L21. EE2
batch reactors, MBR technology appears as a hopeful
occurrence in the aquatic environment can be linked
solution for the improvement of EDCs removal, and,
to insufficient removal in wastewater treatment
more precisely, EE2. Alternative treatments such as
plants. The focus of this review is to consider opti-
mum treatment processes for removal of EE2. The
MnO2, and sand reactor or ozonation were tested in
main problem of EE2 is concentrations often below
the laboratory and were shown to be inadequate.
17a-Ethinylestradiol: AnEndocrine Disrupter of GreatConcern. Analytical Methodsand Removal Processes Appliedto Water Purification. A ReviewLudiwine Clouzot,a Benoıˆt Marrot,a Pierre Doumenq,b Nicolas Rochea
a LM2P2, UMR CNRS 6181, Laboratoire de Me´canique, Mode´lisation et Proce´de´s Propres, Universite´ PaulCe´zanne Aix-Marseille 3, Europoˆle de l’Arbois, Baˆt Lae¨nnec hall C BP 80, 13545 Aix-en-provenceCedex 4; benoit.marrot@univ-cezanne.fr (for correspondence)
b SCC-F-QE, UMR 6171, Laboratoire de Chimie Analytique de l’Environnement, Universite´ Paul Ce´zanneAix-Marseille 3, Europoˆle de l’Arbois, Baˆt Villemin, BP 80, 13545 Aix-en-provence Cedex 4
Published online 16 June 2008 in Wiley InterScience (www.interscience.wiley.com). DOI 10.1002/ep.10291
The xenobiotic 17a-ethinylestradiol, an oral con-
ing microorganisms that are responsible for EE2 bio-
traceptive component, is an endocrine disrupter
degradation. Among bioprocesses, i.e., AS, membrane
(EDC) of great concern, with fish feminization
bioreactors (MBRs), biofilm reactors, and sequencing
induced for concentrations as low as ng L21. EE2
batch reactors, MBR technology appears as a hopeful
occurrence in the aquatic environment can be linked
solution for the improvement of EDCs removal, and,
to insufficient removal in wastewater treatment
more precisely, EE2. Alternative treatments such as
plants. The focus of this review is to consider opti-
mum treatment processes for removal of EE2. The
MnO2, and sand reactor or ozonation were tested in
main problem of EE2 is concentrations often below
the laboratory and were shown to be inadequate.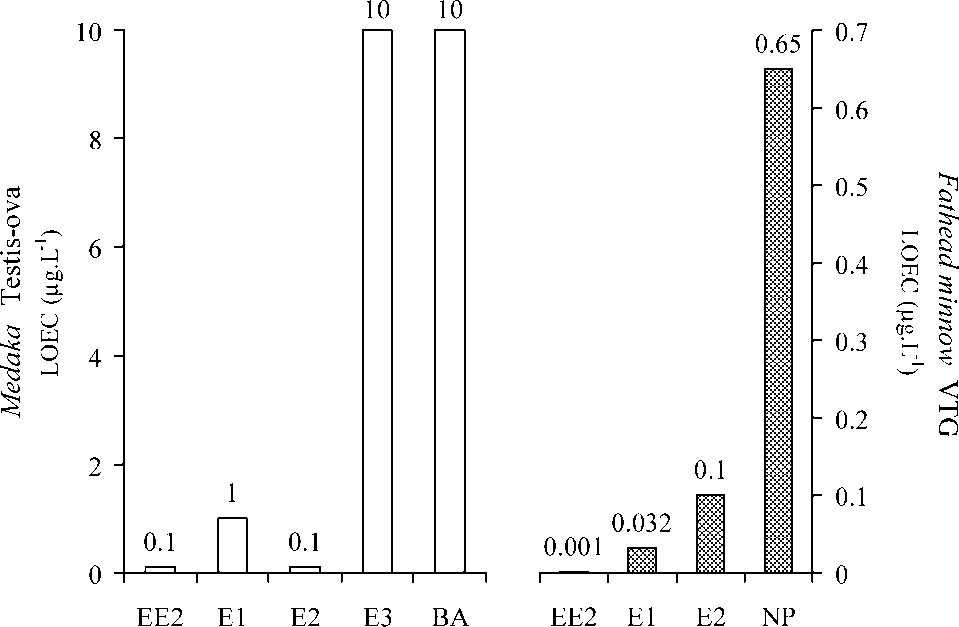 it has at least one of the following characteristics: (i)it is present in environment at high concentrations,(ii) it is persistent and bioaccumulative, or (iii) it isconstantly released into environment [1]. Endocrinedisruptions were highlighted in wildlife, e.g., fishes,mammals, birds, reptiles, amphibians, and inverte-brates [2–4]. Estrogenic responses were first observedin caged trout exposed to sewage effluents [5]. Subse-quently, among many fish species, feminization proc-esses such as testes malformation or intersex fish,with oocytes in the testes, were detected downstreamof municipal sewage effluents: flounder [6–8], Medi-terranean swordfish [9, 10], Rainbow trout [11], roach[12, 13], or cyprinids [14, 15]. Therefore, endocrinedisruptions in the aquatic ecosystem can be linked to
Figure 1. Endocrine disruptions in Medaka and Fat-
EDCs release from waste water treatment plants
head minnow by hormones and alkylphenols. LOEC,
lowest tested concentration at which noted effect
EDCs are composed of a wide range of molecules
occurred; left, testis-ova in Medaka; right, vitellogenin
such as chlorinated pesticides, phthalates, alkylphe-
(VTG) synthesis in fathead minnow. Medaka, adapted
nols, natural and synthetic hormones. EDCs impact
from [21]; Fathead minnow: EE2 [22]; E1 and E2 [23];
on fish populations were previously reviewed by
Mills and Chichester [16]. Laboratory experimentsdemonstrated EDCs in
mainly estrogenic. In surface water, natural estrogenssuch as estrone (E1), 17a-estradiol (E2), estriol (E3),
doses (1–1000 lg kg21) of EE2 in male Rainbow trout
showed VTG synthesis until 720 times more than E2
(EE2), widely used in human oral contraceptives,
[5]. Besides, concentrations as low as 0.1 ng L21 of
were detected in the low ng L21 range. Despite trace
EE2 were shown to cause significant rise in plasma
concentrations, hormones contribute largely to the
VTG. A food-web model of aquatic organisms in river
surface water estrogenicity [17], with 35–50% due to
systems also suggests bioaccumulation of EE2 by
the xenobiotic EE2 [18]. Natural steroids have relative
fishes [27]. Ecotoxicologic studies classified EE2 as at
potency about one million times more than pesticides
least R51/53 that means ‘‘toxic to aquatic organisms
and 300,000 times more than p-nonylphenol (NP)
and may cause long-term effects in the aquatic envi-
[19]. Male Rainbow trouts (Oncorhynchus mykiss),
undergoing sexual maturation, were exposed at 3
EE2 occurrence in the aquatic environment comes
weeks to EE2, NP, and octylphenol (OP) [20]. While
from WWTP effluents, due to insufficient removal
fish exposed to 30 lg L21 of NP and OP showed sig-
during water purification. With regard to endocrine
nificant reduction in testicular growth, males exposed
disruptions caused by EE2, the focus of this review is
to 2 ng L21 of EE2 showed some disruption. Intersex
to consider optimum treatment processes for removal
gonads (testis-ova) were observed in male Medakas
of the synthetic hormone from sewage effluent dis-
(Oryzias latipes) exposed at 100 days post-hatch to
charges. The major issue of EE2 is its occurrence at
EE2, E1, E2, E3, and bisphenol A (BA) (see Figure 1).
it has at least one of the following characteristics: (i)it is present in environment at high concentrations,(ii) it is persistent and bioaccumulative, or (iii) it isconstantly released into environment [1]. Endocrinedisruptions were highlighted in wildlife, e.g., fishes,mammals, birds, reptiles, amphibians, and inverte-brates [2–4]. Estrogenic responses were first observedin caged trout exposed to sewage effluents [5]. Subse-quently, among many fish species, feminization proc-esses such as testes malformation or intersex fish,with oocytes in the testes, were detected downstreamof municipal sewage effluents: flounder [6–8], Medi-terranean swordfish [9, 10], Rainbow trout [11], roach[12, 13], or cyprinids [14, 15]. Therefore, endocrinedisruptions in the aquatic ecosystem can be linked to
Figure 1. Endocrine disruptions in Medaka and Fat-
EDCs release from waste water treatment plants
head minnow by hormones and alkylphenols. LOEC,
lowest tested concentration at which noted effect
EDCs are composed of a wide range of molecules
occurred; left, testis-ova in Medaka; right, vitellogenin
such as chlorinated pesticides, phthalates, alkylphe-
(VTG) synthesis in fathead minnow. Medaka, adapted
nols, natural and synthetic hormones. EDCs impact
from [21]; Fathead minnow: EE2 [22]; E1 and E2 [23];
on fish populations were previously reviewed by
Mills and Chichester [16]. Laboratory experimentsdemonstrated EDCs in
mainly estrogenic. In surface water, natural estrogenssuch as estrone (E1), 17a-estradiol (E2), estriol (E3),
doses (1–1000 lg kg21) of EE2 in male Rainbow trout
showed VTG synthesis until 720 times more than E2
(EE2), widely used in human oral contraceptives,
[5]. Besides, concentrations as low as 0.1 ng L21 of
were detected in the low ng L21 range. Despite trace
EE2 were shown to cause significant rise in plasma
concentrations, hormones contribute largely to the
VTG. A food-web model of aquatic organisms in river
surface water estrogenicity [17], with 35–50% due to
systems also suggests bioaccumulation of EE2 by
the xenobiotic EE2 [18]. Natural steroids have relative
fishes [27]. Ecotoxicologic studies classified EE2 as at
potency about one million times more than pesticides
least R51/53 that means ‘‘toxic to aquatic organisms
and 300,000 times more than p-nonylphenol (NP)
and may cause long-term effects in the aquatic envi-
[19]. Male Rainbow trouts (Oncorhynchus mykiss),
undergoing sexual maturation, were exposed at 3
EE2 occurrence in the aquatic environment comes
weeks to EE2, NP, and octylphenol (OP) [20]. While
from WWTP effluents, due to insufficient removal
fish exposed to 30 lg L21 of NP and OP showed sig-
during water purification. With regard to endocrine
nificant reduction in testicular growth, males exposed
disruptions caused by EE2, the focus of this review is
to 2 ng L21 of EE2 showed some disruption. Intersex
to consider optimum treatment processes for removal
gonads (testis-ova) were observed in male Medakas
of the synthetic hormone from sewage effluent dis-
(Oryzias latipes) exposed at 100 days post-hatch to
charges. The major issue of EE2 is its occurrence at
EE2, E1, E2, E3, and bisphenol A (BA) (see Figure 1).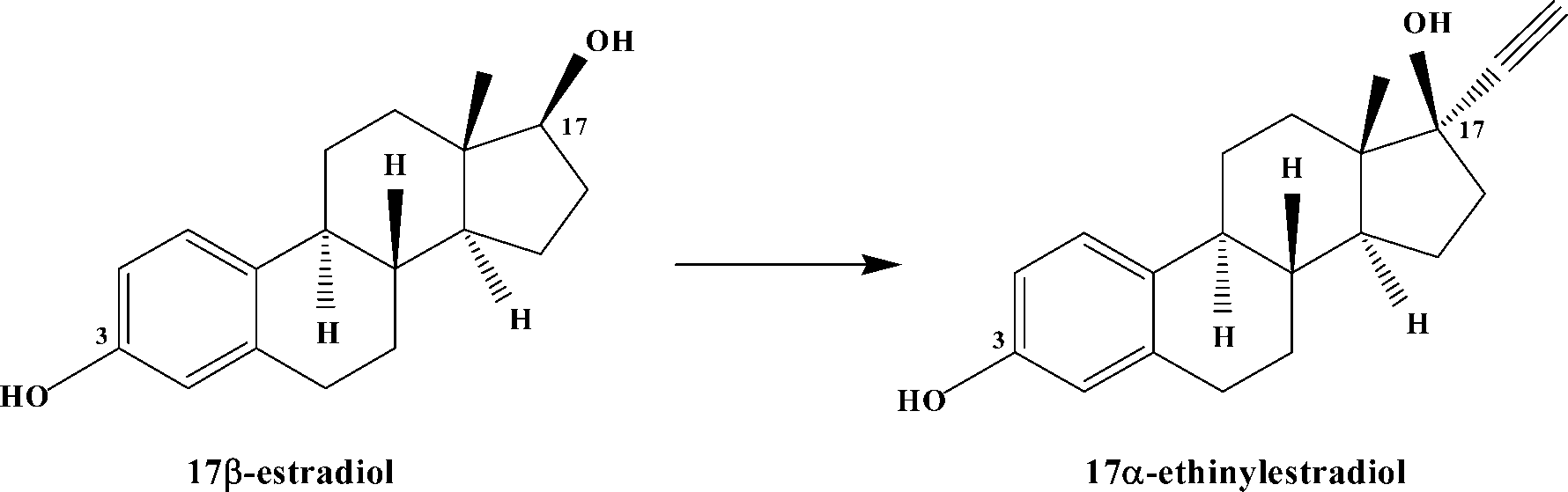 Figure 2. 17a-ethinylestradiol engineered from the natural hormone 17b-estradiol.
Figure 2. 17a-ethinylestradiol engineered from the natural hormone 17b-estradiol.