Microsoft word - festivalsukkot.htm
The Festival of Sukkot begins on Tishri 15, the fifth day after YomKippur. It is quite a drastic transition, from one of the most solemnholidays in our year to one of the most joyous. This festival is sometimes referred to as Zeman Simkhateinu , the Seasonof our Rejoicing. Sukkot lasts for seven days. The two days following thefestival are separate holidays, Shemini Atzeret and Simkhat Torah, bu
 The Journal of Wildlife Management; DOI: 10.1002/jwmg.445
Differential Physiological Responses to PreyAvailability by the Great Egret and White Ibis
GARTH HERRING,1,2 Department of Biological Sciences, Florida Atlantic University, 777 Glades Road, Boca Raton, FL 33431, USA
DALE E. GAWLIK, Department of Biological Sciences, Florida Atlantic University, 777 Glades Road, Boca Raton, FL 33431, USA
ABSTRACT In long-lived species, the balance between the benefits of reproduction and the costs fromreduced survival or productivity is particularly challenging in dynamic environments like wetlands, wherefood levels vary greatly year to year. Some wetland species exhibit changes in reproductive strategies inresponse to food availability but whether physiological responses function in a similar manner is unclear. Wecompared the pre-breeding physiological responses (fecal corticosterone [FCORT], heat shock protein 60[HSP60], and mass) of 2 species of wading birds with contrasting foraging strategies (great egret [Ardea alba],an exploiter, and white ibis [Eudocimus albus], a searcher) during years with contrasting levels of preyavailability. Both species were in good physiological condition, with low levels of HSP60 and FCORT,during a year with high prey availability (2006). In a contrasting year with lesser prey availability (2007),HSP60 and FCORT concentrations indicated that ibis physiological condition was reduced, whereas egretsshowed little change. Egrets and male ibis increased body mass, whereas female ibis decreased mass, in theyear with low prey availability. Although poorly understood, we hypothesize that the differential responsebetween female ibis and the others is associated with differential investment strategies based on long-termcosts of reproduction. Model results identified prey availability and the 2-week water recession rate as theprimary habitat variables that were associated with the physiological condition of white ibises, whereas greategret physiological condition was influenced mostly by 2-week water recession rate. Our results support thehypothesis that prey availability and hydrological factors play crucial roles in regulating populations of wadingbirds in the Florida Everglades. The results of this study show a more complete pathway by which hydrologicpatterns affect wading birds, and it suggests that ibis are more sensitive to habitat conditions than are egrets.
The Journal of Wildlife Management; DOI: 10.1002/jwmg.445
Differential Physiological Responses to PreyAvailability by the Great Egret and White Ibis
GARTH HERRING,1,2 Department of Biological Sciences, Florida Atlantic University, 777 Glades Road, Boca Raton, FL 33431, USA
DALE E. GAWLIK, Department of Biological Sciences, Florida Atlantic University, 777 Glades Road, Boca Raton, FL 33431, USA
ABSTRACT In long-lived species, the balance between the benefits of reproduction and the costs fromreduced survival or productivity is particularly challenging in dynamic environments like wetlands, wherefood levels vary greatly year to year. Some wetland species exhibit changes in reproductive strategies inresponse to food availability but whether physiological responses function in a similar manner is unclear. Wecompared the pre-breeding physiological responses (fecal corticosterone [FCORT], heat shock protein 60[HSP60], and mass) of 2 species of wading birds with contrasting foraging strategies (great egret [Ardea alba],an exploiter, and white ibis [Eudocimus albus], a searcher) during years with contrasting levels of preyavailability. Both species were in good physiological condition, with low levels of HSP60 and FCORT,during a year with high prey availability (2006). In a contrasting year with lesser prey availability (2007),HSP60 and FCORT concentrations indicated that ibis physiological condition was reduced, whereas egretsshowed little change. Egrets and male ibis increased body mass, whereas female ibis decreased mass, in theyear with low prey availability. Although poorly understood, we hypothesize that the differential responsebetween female ibis and the others is associated with differential investment strategies based on long-termcosts of reproduction. Model results identified prey availability and the 2-week water recession rate as theprimary habitat variables that were associated with the physiological condition of white ibises, whereas greategret physiological condition was influenced mostly by 2-week water recession rate. Our results support thehypothesis that prey availability and hydrological factors play crucial roles in regulating populations of wadingbirds in the Florida Everglades. The results of this study show a more complete pathway by which hydrologicpatterns affect wading birds, and it suggests that ibis are more sensitive to habitat conditions than are egrets.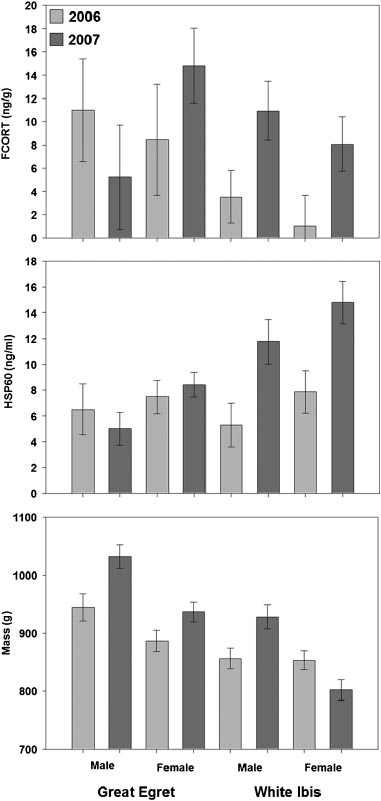 variables of influence for great egret FCORT metabolitelevels had beta coefficient estimates with 95% confidenceintervals that overlapped zero, suggesting none of them had alarge effect (Table 2). Great egrets had similar FCORTconcentrations between the year with high prey availabilityand the year with low prey availability (Fig. 1).
variables of influence for great egret FCORT metabolitelevels had beta coefficient estimates with 95% confidenceintervals that overlapped zero, suggesting none of them had alarge effect (Table 2). Great egrets had similar FCORTconcentrations between the year with high prey availabilityand the year with low prey availability (Fig. 1).