Microsoft word - cv_2012.doc
Yasuyuki Ishikawa E-mail: yishikawa@uprrp.edu Date of Birth: Oct. 10, 1947(naturalized U.S. citizen): Marital Status: Married, one child Education Tokyo Institute of Technology, Japan, B.S. in Chemistry 1971 University of Iowa, Iowa City, Iowa, Ph.D. in Chemistry 1976 Employment Professor of Chemistry University of Puerto Rico, Rio Piedras IMS Visiting Professor University of Puerto Rico, Ri
 Tenofovir Disoproxil Fumarate for Preventionof HIV Infection in Women: A Phase 2, Double-Blind, Randomized, Placebo-Controlled Trial
Leigh Peterson1*, Doug Taylor1, Ronald Roddy2, Ghiorghis Belai1, Pamela Phillips1, Kavita Nanda1,
Robert Grant3,4, Edith Essie Kekawo Clarke5, Anderson Sama Doh6, Renee Ridzon7, Howard S. Jaffe8,
1 Family Health International, Durham, North Carolina, United States of America, 2 Duke Clinical Research Institute, Durham, North Carolina, United
States of America, 3 University of California San Francisco, San Francisco, California, United States of America, 4 J. David Gladstone Institutes, San
Francisco, California, United States of America, 5 Ghana Health Service, Ministry of Health, Accra, Ghana, 6 University of Yaounde´, Yaounde´, Cameroon,
7 Bill and Melinda Gates Foundation, Seattle, Washington, United States of America, 8 Gilead Sciences, Foster City, California, United States of America
Objectives: The objective of this trial was to investigate the safety and preliminary
effectiveness of a daily dose of 300 mg of tenofovir disoproxil fumarate (TDF) versus placebo in
Foundation to Family HealthInternational (FHI). The views expressed
in this article do not necessarily reflectthose of FHI or the Bill and Melinda GatesFoundation. Gilead Sciences provided the
Design: This was a phase 2, randomized, double-blind, placebo-controlled trial.
Tenofovir Disoproxil Fumarate for Preventionof HIV Infection in Women: A Phase 2, Double-Blind, Randomized, Placebo-Controlled Trial
Leigh Peterson1*, Doug Taylor1, Ronald Roddy2, Ghiorghis Belai1, Pamela Phillips1, Kavita Nanda1,
Robert Grant3,4, Edith Essie Kekawo Clarke5, Anderson Sama Doh6, Renee Ridzon7, Howard S. Jaffe8,
1 Family Health International, Durham, North Carolina, United States of America, 2 Duke Clinical Research Institute, Durham, North Carolina, United
States of America, 3 University of California San Francisco, San Francisco, California, United States of America, 4 J. David Gladstone Institutes, San
Francisco, California, United States of America, 5 Ghana Health Service, Ministry of Health, Accra, Ghana, 6 University of Yaounde´, Yaounde´, Cameroon,
7 Bill and Melinda Gates Foundation, Seattle, Washington, United States of America, 8 Gilead Sciences, Foster City, California, United States of America
Objectives: The objective of this trial was to investigate the safety and preliminary
effectiveness of a daily dose of 300 mg of tenofovir disoproxil fumarate (TDF) versus placebo in
Foundation to Family HealthInternational (FHI). The views expressed
in this article do not necessarily reflectthose of FHI or the Bill and Melinda GatesFoundation. Gilead Sciences provided the
Design: This was a phase 2, randomized, double-blind, placebo-controlled trial.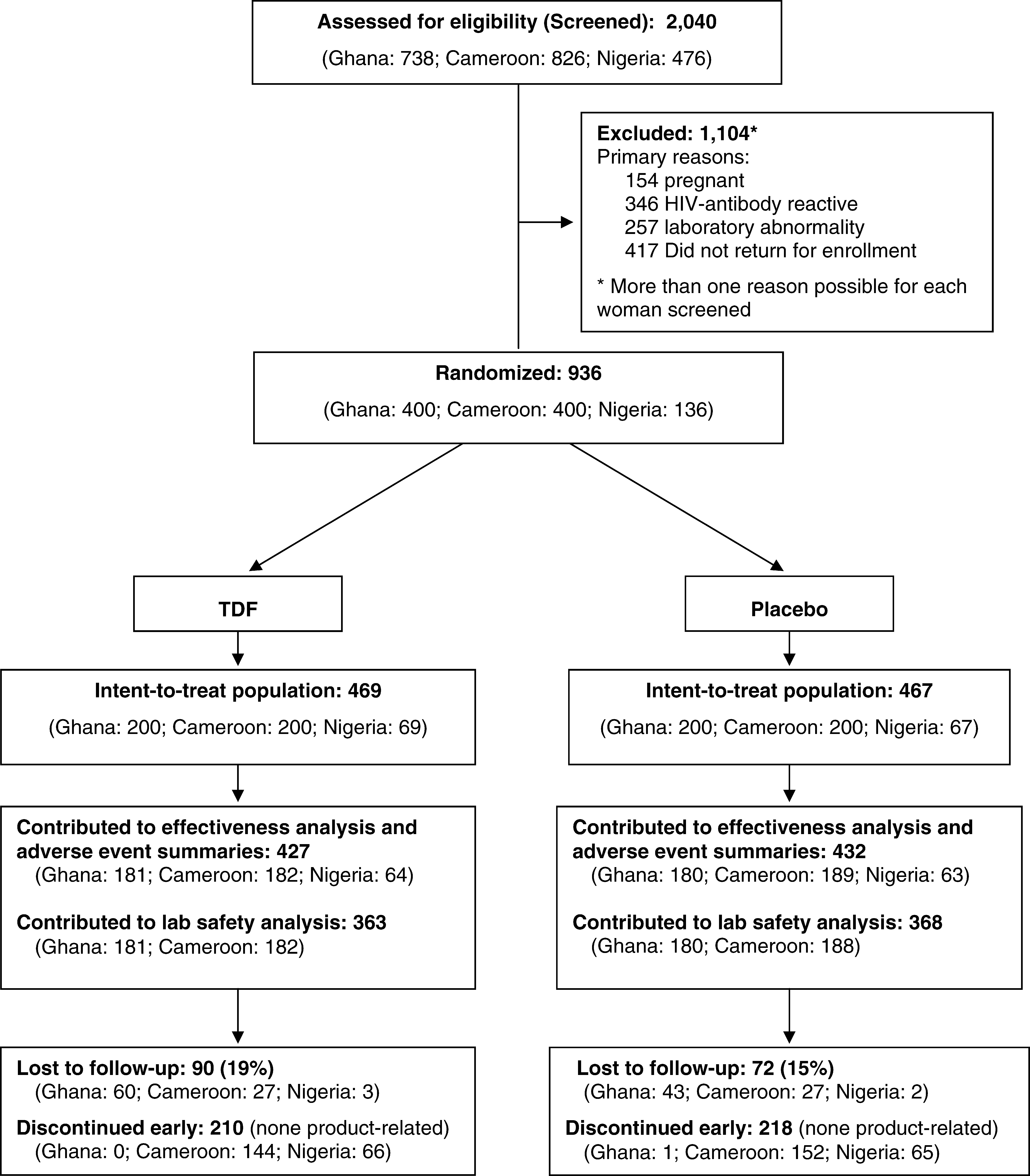 used for no more than 69% of all study days (78% in
a grade 3þ decrease in phosphorus (,1.5 mg/dl), which
Cameroon, 68% in Ghana, and 50% in Nigeria). Excluding
spontaneously resolved to normal within 3 mo (study product
time off product due to pregnancy, drug was used for no
was not withdrawn). No participants in either group had a
more than 74% of study days. Missed/late clinic visits and
grade 2þ creatinine elevation (.2.0 mg/dl). We did not find
pregnancy were the principal documented reasons for time
significant differences in laboratory abnormalities between
treatment groups when the data were stratified by site,although significantly more grade 1 AST and phosphorus
abnormalities occurred in Ghana than in Cameroon, and
Hepatic and renal function. Analysis of hepatic and renal
significantly more grade 1 creatinine abnormalities occurred
function was restricted to data from Ghana and Cameroon,
which resulted in 210.2 person-years of follow-up in the TDF
Among the 56 participants who tested positive for HBsAg,
group and 217.6 person-years in the placebo group. We did
23 were in the TDF group and 33 in the placebo group. The
not find significant differences in the primary safety
mean and median ALT and AST levels were not significantly
endpoints between treatment groups (Table 2). Specifically,
different between groups immediately before or after
none of the 363 participants assigned to TDF in Ghana or
discontinuation of study drug. Four ALT/AST abnormalities
Cameroon had grade 3 or higher (.170 U/l) ALT or AST
(none over grade 1 [.42 U/l]) occurred within 3 mo after
elevations before product withdrawal, whereas two and three
discontinuation of study drug in HBsAg-positive participants;
of the 368 participants in the placebo group had grade 3þ
one was in the TDF group and three were in the placebo
ALT and AST elevations, respectively. The percentage of
grade 1 or higher (.42 U/l) ALT and AST abnormalities was
In Cameroon, study drug was stopped before the imple-
greater in the TDF group, but the difference did not achieve
mentation of the protocol amendment that included HBsAg
statistical significance. One participant in the TDF group had
testing. We therefore monitored liver function for several
If have been pregnant, number of pregnancies
Had any sexually transmitted infection in past 6 mo, n (%)
months after product withdrawal in all participants, regard-
participant by the site investigator or study clinician because
less of HBV status. The mean and median ALT and AST levels
of an AE or abnormal laboratory result.
used for no more than 69% of all study days (78% in
a grade 3þ decrease in phosphorus (,1.5 mg/dl), which
Cameroon, 68% in Ghana, and 50% in Nigeria). Excluding
spontaneously resolved to normal within 3 mo (study product
time off product due to pregnancy, drug was used for no
was not withdrawn). No participants in either group had a
more than 74% of study days. Missed/late clinic visits and
grade 2þ creatinine elevation (.2.0 mg/dl). We did not find
pregnancy were the principal documented reasons for time
significant differences in laboratory abnormalities between
treatment groups when the data were stratified by site,although significantly more grade 1 AST and phosphorus
abnormalities occurred in Ghana than in Cameroon, and
Hepatic and renal function. Analysis of hepatic and renal
significantly more grade 1 creatinine abnormalities occurred
function was restricted to data from Ghana and Cameroon,
which resulted in 210.2 person-years of follow-up in the TDF
Among the 56 participants who tested positive for HBsAg,
group and 217.6 person-years in the placebo group. We did
23 were in the TDF group and 33 in the placebo group. The
not find significant differences in the primary safety
mean and median ALT and AST levels were not significantly
endpoints between treatment groups (Table 2). Specifically,
different between groups immediately before or after
none of the 363 participants assigned to TDF in Ghana or
discontinuation of study drug. Four ALT/AST abnormalities
Cameroon had grade 3 or higher (.170 U/l) ALT or AST
(none over grade 1 [.42 U/l]) occurred within 3 mo after
elevations before product withdrawal, whereas two and three
discontinuation of study drug in HBsAg-positive participants;
of the 368 participants in the placebo group had grade 3þ
one was in the TDF group and three were in the placebo
ALT and AST elevations, respectively. The percentage of
grade 1 or higher (.42 U/l) ALT and AST abnormalities was
In Cameroon, study drug was stopped before the imple-
greater in the TDF group, but the difference did not achieve
mentation of the protocol amendment that included HBsAg
statistical significance. One participant in the TDF group had
testing. We therefore monitored liver function for several
If have been pregnant, number of pregnancies
Had any sexually transmitted infection in past 6 mo, n (%)
months after product withdrawal in all participants, regard-
participant by the site investigator or study clinician because
less of HBV status. The mean and median ALT and AST levels
of an AE or abnormal laboratory result.